What is hybridization? Explain sp² hybridization with example.
"The process of mixing orbitals of different energy and shape to form set of new orbital of the same energy and same shape is called Hybridization and orbitals obtained are called hybrid orbitals".
sp²-Hybridization
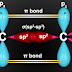
The process of mixing one 's' and two 'p' orbitals to form three equivalent sp² hybrid orbitals is called sp2-Hybridization.
Each sp² orbital consists of 's' and 'p' in the ratio of 1 : 2 respectively.
sp²-hybrid orbitals lie at the angle of 120° in a plane. The geometry of the molecules is trigonal planar.
Formation of Ethylene or Ethane (C2H4) Molecule
Electron configuration of C (6) = 1s ⇵, 2s ⇵, 2px↑, 2py↑, 2pz
Excited state = 1s ⇵, 2s ↑, 2px ↑, 2py ↑, 2pz ↑
One s and two p orbitals intermix to form three hybrid (sp²) orbitals. The geometry of molecules depends upon the number of hybrid orbitals. Hybrid orbitals are trigonal planar and are oriented at the angle of 120°. Each atom is left with one half filled p-orbital perpendicular to the planar sp² hybrid orbitals. Each carbon atom undergeos sp² -s, overlaps with two hydrogen atoms and sp² -sp² overlap between themselves to form sigma bonds. These overlaps lead to the following shapes. The partially filled p-orvbitals undergo overlap sideways to form a pi-bond. So, a pi-bond is formed by the sideways overlap of two half filled co-planar p-orbital in such a way that the probability of finding the electron is maximum perpendicular to the line joining the two should be made clear that a π-bond is formed between two atoms only, when the with a sigma bond.
 Reviewed by SaQLaiN HaShMi
on
7:52 AM
Rating:
Reviewed by SaQLaiN HaShMi
on
7:52 AM
Rating:







No comments: